
The periodic table continues to evolve with the progress of science. The periodic table and law are now a central and indispensable part of modern chemistry. Seaborg's discovery that the actinides were in fact f-block rather than d-block elements. A recognisably modern form of the table was reached in 1945 with Glenn T. It was explained early in the 20th century, with the discovery of atomic numbers and associated pioneering work in quantum mechanics both ideas serving to illuminate the internal structure of the atom. The periodic law was recognized as a fundamental discovery in the late 19th century. As not all elements were then known, there were gaps in his periodic table, and Mendeleev successfully used the periodic law to predict some properties of some of the missing elements. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869 he formulated the periodic law as a dependence of chemical properties on atomic mass. Nonmetallic character increases going from the bottom left of the periodic table to the top right.

Metallic character increases going down a group and decreases from left to right across a period.

Vertical, horizontal and diagonal trends characterize the periodic table. Elements in the same group tend to show similar chemical characteristics.
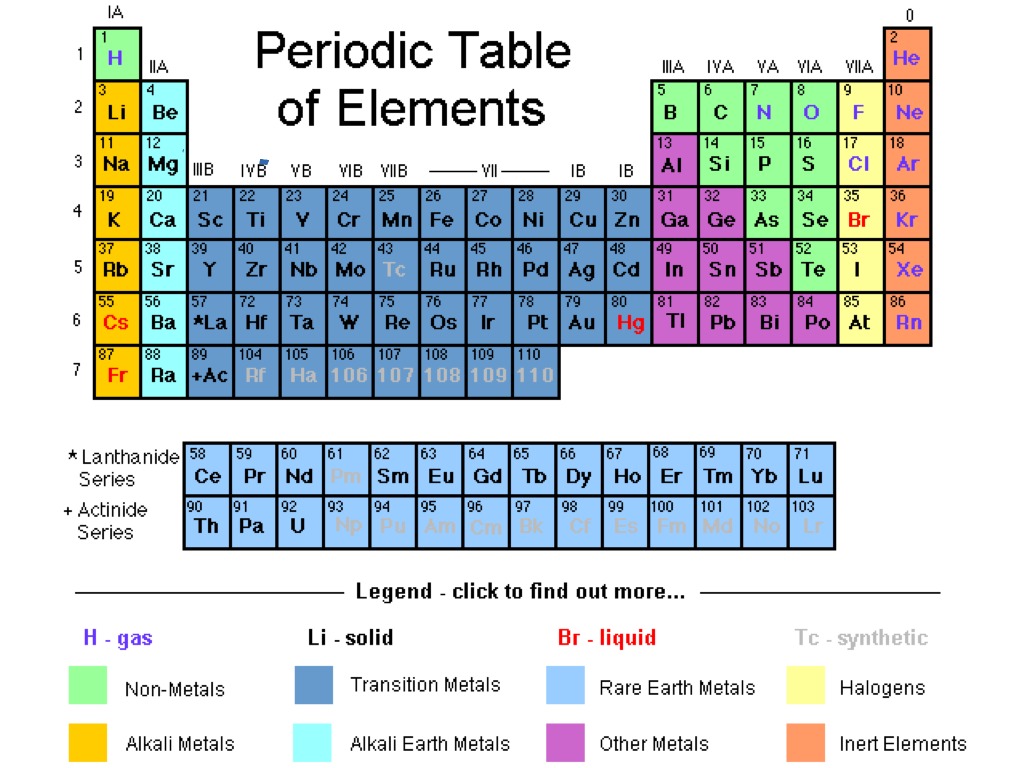
The table is divided into four roughly rectangular areas called blocks. It is a depiction of the periodic law, which says that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. It is an organizing icon of chemistry and is widely used in physics and other sciences. The periodic table, also known as the periodic table of the elements, arranges the chemical elements into rows (" periods") and columns (" groups").


 0 kommentar(er)
0 kommentar(er)
